Vaccine against covid: why several European countries do not want to give Oxford vaccine to the elderly
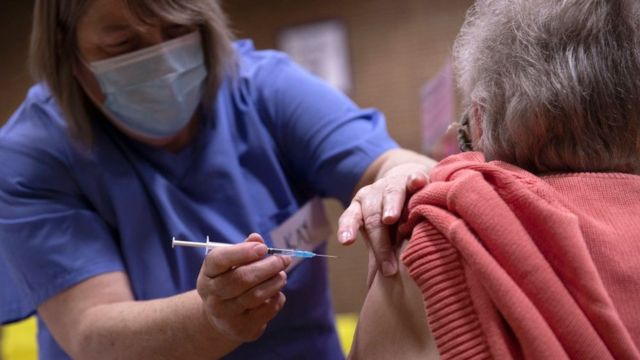
France is one of several EU countries that do not recommend the Oxford immunizer for people over 65
Hours after the European Medicines Agency (EMA) approved the Oxford-AstraZeneca vaccine on January 29 for use in all age groups in the European Union, French President Emmanuel Macron said the immunizer was " almost ineffective "for people over 65.
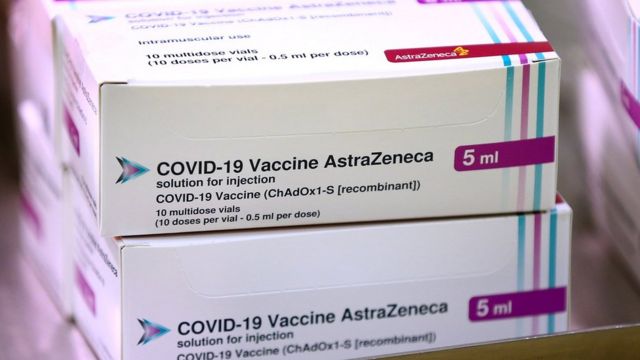
Other European countries have adopted a similar position: Germany, Austria, Sweden and Poland only recommend it for children under 65, and Italy and Belgium for children under 55.
UK and several other countries, including Brazil, India, Mexico and Argentina, have approved use of the AstraZeneca vaccine for all age groups
But the UK and several other countries, including Brazil, India, Mexico and Argentina, have approved its use for all age groups.
"Current evidence does not suggest any lack of protection against covid-19 in people aged 65 and over who received the AstraZeneca vaccine," said the chief executive of the UK Medicines and Health Products Regulatory Agency (MHRA), June Raine, through a statement.
"The data we have shows that the vaccine produces a strong immune response in people over 65 and that it is safe."
In a note, the Oswaldo Cruz Foundation (Fiocruz), responsible for importing and manufacturing the Oxford-AstraZeneca vaccine in Brazil, stated that "the evidence presented so far and published in specialized scientific journals confirms safety and immunogenicity (production of antibodies ) of the Oxford-AstraZeneca vaccine in the elderly ".
In addition, Fiocruz says that the National Health Surveillance Agency (Anvisa) pointed out that the number of participants aged 65 and over "is still small and the estimate of effectiveness is not yet statistically significant", but that ongoing studies should be carried out. available soon with these consolidated data. And he reiterates: "The evidence presented already confirms the safety and immunogenicity (production of antibodies) of the vaccine for the elderly".
What does the evidence show?
Ultimately, decisions are limited to individual interpretations of the available data.
During clinical trials, vaccines are administered to thousands of people of different age groups, ethnicities and health conditions.
Others receive a placebo - which is effectively an injection that does not contain a vaccine - for comparison.
Scientists then expect a number of participants to contract the virus to see who has been infected. If the majority of them are from the group that received the placebo, this suggests that the vaccine is effective.
The problem, for some European regulators, is that they think that few trial participants over the age of 65 have contracted the virus to draw conclusions about the vaccine's effectiveness. This is because only two of the 660 people in this age group have been infected.
French President Emmanuel Macron said Oxford immunizer was "almost ineffective" for people over 65
Other companies, like Pfizer, included more older people at the start of their tests, so they have more data available.
The MHRA recommendation says that the number of older people who contracted the virus in the AZ trial was "too small to draw conclusions about the effectiveness".
Therefore, French, German and other agencies are focusing on this fact.
"Their assessment is that the effectiveness has not yet been demonstrated for people over 65. They did not say that the vaccine is ineffective for people over 65," said Jim Naismith, professor of structural biology at the University of Oxford in the United Kingdom.
In other words, they are not arguing that the vaccine is 'almost ineffective' in older people - as President Macron said. But they are waiting for more information, which should be available in the coming weeks.
"Scientists often disagree about how much evidence is needed for any new breakthrough and there is always more data to be obtained," explains Naismith. "Usually, this all happens outside the reach of the media vision and not in a pandemic, but these debates are an important part of the scientific process."
Immune response
Those who are most confident in the vaccine's effectiveness in people over 65 are looking at other parts of the data.
For example, during an early stage of clinical testing, the vaccine has been shown to stimulate an immune response in older age groups, similar to that seen in younger people.
This means that blood tests on vaccinated people have found evidence of antibodies that help fight disease caused by the virus.
Mary Ramsay, head of immunizations at Public Health England (PHE), the British government's health agency, said that although "there were few cases in older people in AstraZeneca tests to see accurate levels of protection in this group ... data on immune responses were very reassuring ".
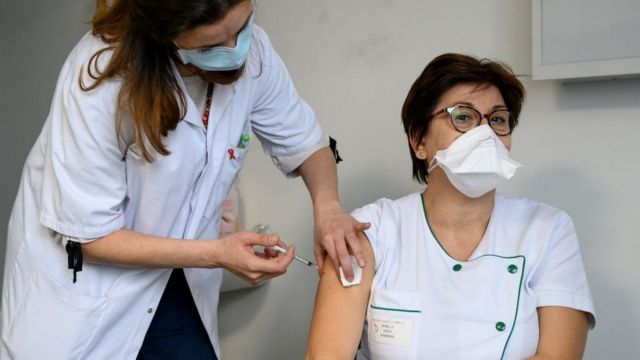
Most importantly, there is no disagreement between health regulators over the safety of the vaccine, which has proven safe for use by people over 18.
All vaccine companies are also testing, including vaccines that have already been approved. This will help to create even more accurate information about the effectiveness of different vaccines in different groups or with different dosage sizes.
Oxford vaccine developers say the results of an experiment with 2,000 adults over 55 in the UK will be available soon, and another in the United States in older age groups is also expected to provide data soon.
Blood tests on vaccinated people found evidence of antibodies that help fight coronavirus disease
What did the European authorities say?
The European agency, EMA, is being a little less cautious than some of the national regulators in EU countries.
The organization said in its recommendation on January 29 that "there are still not enough results in older participants (over 55 years old) to provide a picture of how well the vaccine will work in this group".
However, he added that "EMA scientists believe that the vaccine can be used in the elderly".
Prior to EMA approval, Germany's public health agency, Robert Koch Institute, said there was "insufficient data" currently available on the vaccine's effectiveness in people over 65 and recommended its use in people between 18 and 64 years old .
The BBC contacted the French Agency for the Safety of Medicines and Health Products (ANSM) about the statement by French President Emmanuel Macron, as well as his recommendation for the use of the vaccine in people over 65, but has not obtained response until the closing of this report.
But an official at the Elysee Palace (official residence of the President) said: "Our strategy is to be efficient and protect our citizens. The debate on the vaccination schedule, its speed, is legitimate, as is the debate on health and safety vaccine authorizations. "
However, by the time the Oxford-AZ vaccine is widely available in the EU, it is possible that the views of national regulators who are exercising caution may have changed as more data emerges.


No comments:
Post a Comment