China announces 'conditional' approval of Pfizer's anti-Covid pill
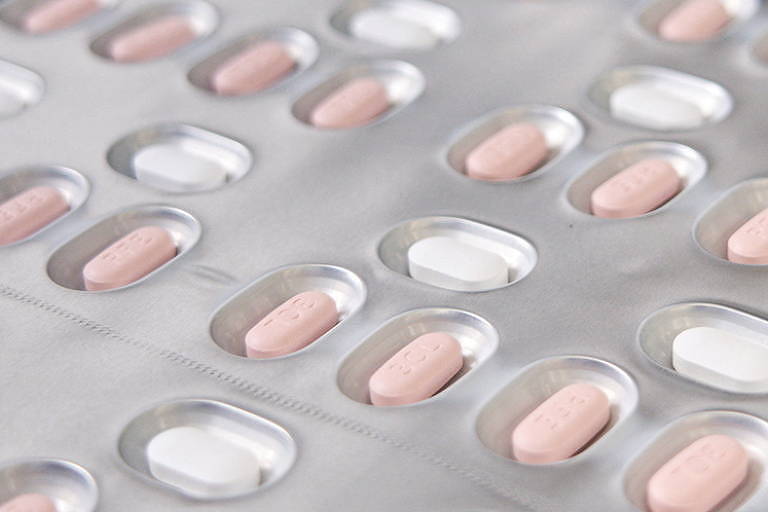
Paxlovid reduces deaths in those at risk of developing severe disease
China's drug regulator said on Saturday it had given "conditional" approval to use the US-based Pfizer 's anti-Covid pill to treat adults with mild to moderate illnesses who may develop severe symptoms. .
The National Medical Devices Administration also requested that more research on the drug be carried out and sent to the entity.
Administered orally, this antiviral treatment is marketed as paxlovid and has been licensed in at least 40 countries, including the United States and Israel. The European Union has allowed its use to member countries while processing their official authorization.

No comments:
Post a Comment