Alzheimer's: good news and three concerns about new drugs
New drugs were the first to slow progression of symptoms
Research into a treatment for Alzheimer's disease, the most common type of dementia, has gone on for a long time without major news. In the past two decades, no new medicine had been released.
And it wasn't for lack of trying: more than a hundred candidates for new treatments were evaluated, but all of them frustrated the expectations of doctors, patients and families.
The scenario changed in 2021, with the approval of the drug aducanumab (from Biogen) by the Food and Drug Administration (FDA), the US regulatory agency.
It is worth mentioning that the release of this drug generated controversy in the scientific community, and subsequent requests for its use in other places (such as Europe and Brazil) were denied.
And one more option may be on the way: at the 2023 Alzheimer's Association International Conference, held a little while ago in the Netherlands, the positive results of studies with donanemab (Eli Lilly) were presented, which was able to stop the progression of Alzheimer's symptoms. illness.
On the one hand, recent advances were celebrated and renewed hopes, by indicating ways to at least delay the loss of memories and reasoning.
On the other hand, experts heard by BBC News Brasil question who will really benefit from the new treatments when they are actually available.
Find out below the details of the medications and the arguments raised by doctors and pharmaceutical companies responsible for the innovations.
And one more option may be on the way: at the 2023 Alzheimer's Association International Conference, held a little while ago in the Netherlands, the positive results of studies with donanemab (Eli Lilly) were presented, which was able to stop the progression of Alzheimer's symptoms. illness.
On the one hand, recent advances were celebrated and renewed hopes, by indicating ways to at least delay the loss of memories and reasoning.
On the other hand, experts heard by BBC News Brasil question who will really benefit from the new treatments when they are actually available.
Find out below the details of the medications and the arguments raised by doctors and pharmaceutical companies responsible for the innovations.
Good news: 'extraordinary' progress
João Fellet tries to understand how Brazilians reached the current degree of division.
episodes
End of Podcast
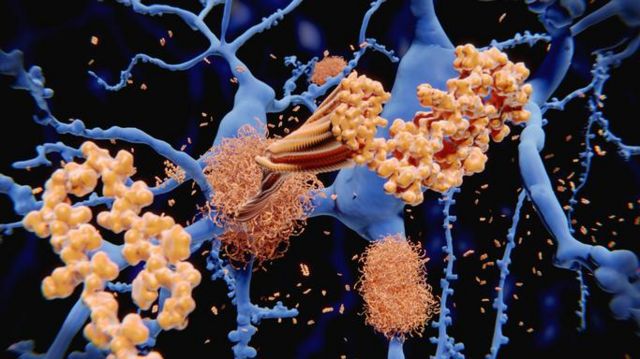
In summary, Alzheimer's disease is marked by two main processes. In the first, there is an accumulation of a protein called beta-amyloid in the spaces between neurons.
Years later, these nerve cells are affected by another protein, known as TAU.
The result of this is the death of neurons, which leads to the progressive appearance of symptoms such as forgetfulness and reasoning difficulties.
But what if it were possible to "sweep" that beta-amyloid from the brain? Would that be able to interrupt the evolution of this disease?
It was precisely from this hypothesis that the first tests with monoclonal antibodies emerged, a pharmacological family that includes aducanumab, lecanemab and donanemab, among other active ingredients.
Such monoclonal antibodies are used not only against Alzheimer's, but there are also approved molecules of this type against various tumors and even against covid-19.
In the universe of dementia, however, the first tests with these drugs ended up frustrated. Some earlier versions of the monoclonal antibodies were even able to clear beta-amyloid from the nervous system, but this did not translate into clinical improvements among the volunteers.
In other words: their brains even had less of this toxic protein, but the impacts on memories and thinking continued to advance in an unbridled way.
But then the experts had another idea. Alzheimer's is a slow, progressive disease — and there is a window of years or even decades between the onset of beta-amyloid accumulation and the appearance of the first symptoms.
What if the drugs were used precisely at this stage, classified as mild cognitive impairment or early dementia?
These studies have been conducted over the last seven to five years, during which time researchers have learned valuable lessons.
The first is that the formation of beta-amyloid skeins in the brain can be broken down into a series of steps. They arise as monomers, evolve into oligomers, and then form fibrils. With the advancement of knowledge, specialists were able to understand in detail what happens in each of these phases.
Complicated names aside, in practice this means that different monoclonal antibodies can act in one phase or another of this process, which supposedly would lead to better or worse results.
"The question was how to interfere in this cascade of events, so that it could be interrupted before the condition became irreversible", contextualizes neurologist Fábio Porto, scientific director of the Brazilian Association of Alzheimer's - Regional São Paulo.
The second learning has to do with the need to make an early diagnosis of the disease. Now, if the idea is to treat individuals who have not even shown symptoms (or are still experiencing very mild discomfort), how can we know who has beta-amyloid aggregates forming in the brain?
The need to identify these individuals led to a veritable revolution in tests to identify Alzheimer's.
Although even today, in doctors' offices, the diagnosis depends on the evaluation of the health professional and the application of a questionnaire, more assertive tests are starting to appear, which are able to quantify the toxic protein in the nervous system.
This can be done, for example, through imaging tests (such as PET/CT), CSF (the collection by puncture of a sample of the liquid present in the spinal cord and brain) and even blood.
Although these tools are still restricted to the research environment and large specialized centers, the tendency is for them to become popular in the coming years.
An exam that evaluates blood plasma to aid in the diagnosis of Alzheimer's, for example, has already been approved by the FDA in the United States.
"And they will certainly be available for research and clinical practice soon in Brazil", predicts physician Ricardo Nitrini, professor of neurology at the Faculty of Medicine of the University of São Paulo (FMUSP).
"But the importance of early diagnosis is not, and should not be, to create stigmas, but rather to allow the advance in methods that allow prevention. We can compare them to exams for the early detection of breast or prostate cancer ", ponders the neurologist.
"The greatest advances in medicine always depend on very early diagnosis and prevention. And we are rapidly approaching this stage of Alzheimer's disease", he adds.
And, it goes without saying, all these technologies only became real from the needs that appeared during tests with monoclonal antibodies and other medications — which, in itself, already represents excellent news.
"Undoubtedly, these new medications represent an extraordinary scientific advance", agrees neurologist Paulo Caramelli, professor at the Department of Internal Medicine at the Federal University of Minas Gerais (UFMG).
"They contribute to validating theories about Alzheimer's and show that, in fact, the beta-amyloid protein plays an important role in the evolution of the disease", he adds.
The accumulation of beta-amyloid proteins (in yellow in the illustration) in neurons (in blue) is one of the processes behind Alzheimer's
Concern 1: 'modest' results
As explained above, the studies that evaluated monoclonal antibodies in the early stages of Alzheimer's had the main objective of checking whether the treatment resulted in clinical improvements — that is, a recovery of cognitive functions or at least a reduction in the rate of worsening.
Tests with lecanemab , for example, involved 1795 participants with mild dementia. Half of them received the medicine, while the other portion took a placebo, a substance with no therapeutic effect. All underwent examinations and cognitive tests to compare the results.
At the end of the 18-month trial, the group using this monoclonal antibody had less beta-amyloid and had a "moderately smaller decline in measures of cognition and function" when compared to those taking a placebo.
With donanemab , the scheme was similar: 1736 volunteers divided into two groups (drug versus placebo) followed for a year and a half.
The results also show a slowing of up to 60% of cognitive decline in those who received the therapy.
But how to translate this information into practice?
"This reduction in the decline means that the patients who took the treatment got worse less than those who took a placebo. But they didn't stop getting worse", answers Porto.
"It was possible to delay the progression of the stages of Alzheimer's disease by about four to six months", adds the doctor.
In other words: the treatment with monoclonal antibodies worked as a kind of brake, which held back for an extra time the evolution of Alzheimer's to the most serious and disabling stages.
"These medications are definitely able to substantially reduce amyloid deposits. This is unequivocal and indisputable. But we still have a modest clinical effect, which may be difficult to measure from an individual point of view", analyzes Caramelli, who is also the coordinator of the International Society for the Advancement of Alzheimer's Research and Treatment (Istaart).
"For people who eventually use these drugs, this is something that will need to be very well explained", points out the neurologist.
BBC News Brasil sought out the pharmaceutical companies responsible for the monoclonal antibodies so that they could position themselves on these points.
For Luiz André Magno, senior medical director at Eli Lilly in Brazil, donanemab opens up "the possibility of delaying the progression of the disease and the cognitive decline of patients".
"When we talk about a disease that has no cure, seeking solutions to reduce the impact it can cause on the lives of patients and their families is the most important and effective thing", he argues.
"This means more time in the less impactful, more functional stages of the disease, as well as a delay in the onset of a later stage of decline. This is extremely valuable for patients themselves and those around them," he says.
In a note, the Eisai laboratory recalled that lecanemab achieved the proposed objectives in clinical studies.
"And these results provide further support for the clinical significance of the improvements seen on cognitive tests. Furthermore, based on information and guidance on clinical significance from experts in Alzheimer's disease and regulatory agencies, Eisai believes that a 25% effect or more is clinically significant", defends the text.
Biogen, responsible for aducanumab, which was not approved for use in Brazil by the National Health Surveillance Agency (Anvisa), declared that it has "a long-term commitment to the Alzheimer's community".
"The bold search for transformative treatments for this incurable disease goes through the unmet need of patients, families, caregivers and the need to reduce the financial burden on health systems. The company also emphasizes that it has a robust development program with a focus on this therapeutic area."
"We are committed to dedicating our efforts to bringing solutions to what is now considered a public health crisis on a global scale. Our science serves humanity, and we will always seek to do what is best for the patient", concludes the text.
About 100,000 Brazilians are diagnosed with Alzheimer's every year
Concern 2: 'Potentially serious' side effects
Clinical trials with the monoclonal antibodies have detected some major adverse events among patients taking the treatment.
On lecanemab, researchers reported side effects classified as "serious." They are: infusion-related reactions (these drugs are given into a vein once a fortnight or a month), swelling and small hemorrhages in the brain (known by the acronym in English ARIA-E), atrial fibrillation (a type of cardiac arrhythmia) , syncope and angina (chest pain).
As for donanemab, the authors also highlight the higher risk of ARIA-E and drug infusion-related reactions. Three volunteers died during the study after complications related to swelling and small hemorrhages in the brain.
"The new drugs had potentially serious side effects", observes Nitrini.
Caramelli points out that some patients with a certain genetic profile are more at risk of developing these adverse events.
"We know that these side effects are more common in people with Alzheimer's who have a specific variant of a gene called APOE", he details.
"Possibly, in clinical use, it will be recommended to carry out a genetic test before taking the decision on whether or not to carry out the treatment", adds the doctor.
Porto agrees and understands that the decision on whether or not to use these drugs will be shared by all those involved.
"We will need to sit down with the family and the patient to show all the evidence, as the barrier between the effectiveness and safety of these drugs is tenuous", he says.
Eisai responded to the report by saying that in the US, where lecanemab is already approved, the drugmaker "has developed an educational initiative to further advance the community's understanding of the management and monitoring of ARIA in the real world."
Bearing in mind that, in this context, ARIA (or amyloid-related imaging abnormalities) are the cerebral edema and microhemorrhages that affected some volunteers in the clinical trials.
"The initiative has an online portal on the detection of ARIA aimed at neurologists, radiologists and other essential health professionals for the management of patients with Alzheimer's disease", informs Eisai.
Magno said, "Eli Lilly is committed to better understanding adverse events for this class of therapies, including patient risk factors and potential mitigants to improve patient safety."
"As a result, Lilly has also initiated the Trailblazer-Alz 6 study, which will help us understand the relationship of adverse events more deeply," he adds.
Swelling and bleeding in the brain was one of the adverse events seen in studies with monoclonal antibodies
Concern 3: 'extremely high' prices
Innovative therapies often come to market with a very high price.
Aducanumab, the first approved Alzheimer's treatment in decades, cost $56,000 a year in the United States. After a while, Biogen announced that it would cut the price in half – and now it sells for US$ 28 thousand (R$ 132 thousand) there.
Lecanemab, on the other hand, costs US$ 26,500 (R$ 123,000) a year for Americans.
And, according to experts heard by BBC News Brasil, the price can be a major impediment to access to monoclonal antibodies by most patients.
"We are talking about extremely high values not only for low- and middle-income countries, like Brazil, but even for high-income countries, like the United States and the United Kingdom", highlights Caramelli.
"In our Constitution it is written that 'health is the right of the people and the duty of the State'. This is a problem at a time when treatments are very expensive", observes Nitrini.
Values like these can be unsustainable even for public and private health systems.
"It's one thing to have a high cost for a rare disease treatment, which affects few people. It's another to have that price for a drug against a disease as common as Alzheimer's", compares Porto,
The Ministry of Health estimates that there are 1.2 million patients with Alzheimer's in the country. Every year, another 100,000 individuals receive this diagnosis in the country.
In response to the report, Eli Lilly said that, "as donanemab is in the process of approval or regulatory submission, that is, it is not officially approved in any country, we cannot yet talk about this topic [the cost of treatment] ".
"We carried out the regulatory submission to the FDA in the second quarter of 2023 and a response is expected by the end of the year, and we are working on the regulatory submission dossier to Anvisa, as well as in other countries", detailed Magno, medical director of the pharmaceutical .
Eisai understands that it is "premature" to talk about the price of lecanemab in Brazil.
"We believe it is our mission to ensure that patients in need have access to innovative medicines and we are committed to ensuring access by taking into account the health system and income level of each country", says the text sent to BBC News Brasil .
New drugs will target not only the beta-amyloid protein, but also TAU and inflammation
What does the future hold?
It is worth emphasizing that these new drugs will not be available to all patients with Alzheimer's: they will only be prescribed for very early cases, since they do not bring gains in the moderate or advanced stages of the disease.
And that even leads us to another discussion: how are patients with dementia in more advanced stages? For now, the drugs available for these cases (such as cholinesterase inhibitors, memantine and antidepressants) act on symptoms, such as agitation or emotional problems.
"In recent years, there has been a shift in focus to early treatment and a significant reduction in the number of studies in the intermediate and advanced stages of Alzheimer's", regrets Caramelli.
But news is expected for the future.
"Certainly, the treatment of Alzheimer's disease will not be done only with antibodies against the beta-amyloid protein", bets Nitrini.
Quelling brain inflammation or clearing TAU protein, other hallmarks of this type of dementia, are other possible therapeutic targets.
"In the not-so-close future, gene therapy or genetics should come into play. Alzheimer's is a predominantly genetic disease", continues the neurologist
"Therapeutic advances will have to include corrections for mutations, which are rare in Alzheimer's, or corrections for polymorphisms [variations in genes], which are common and are beginning to be targets of gene therapy", he says.
For Porto, the future of therapies against this dementia will be similar to what happens today in cancer, in which the line of care changes according to the characteristics of the patient and the particularities of the tumor.
"We can start with a medicine and, when it stops working, move on to a second or third line of options", speculates the doctor.
Caramelli considers that it is almost impossible to find a patient with dementia who is affected only by Alzheimer's. According to him, memory and cognition problems usually have multiple causes.
It is common, for example, to find aggregates of beta-amyloid and TAU (typical of Alzheimer's) together with vascular dementia (when problems in cerebral blood circulation lead to the death of neurons).
"Even if we someday have a 'silver bullet' that eliminates Alzheimer's, it won't solve the problem once and for all," he ponders.
Faced with this evidence, and in the midst of so many valuable technological and scientific advances, the UFMG professor believes that the key to dealing with Alzheimer's will remain in prevention.
"All of us are unfortunately at risk of developing this accumulation of abnormal proteins in the brain," he says.
"What we need to do is become aware and aware that it is possible to prevent some of the risk factors for Alzheimer's. Therefore, controlling high blood pressure, diabetes, cholesterol and weight, exercising, not smoking, seeking a healthy diet and having a cognitive and intellectual activity are fundamental attitudes for the health of the brain", concludes the neurologist.



No comments:
Post a Comment